In an effort to help our PWS community better understand its SCOUT-015 trial, Radius Health has released several resources about the process of the study, trial sites, and about their drug RAD011 a synthetic cannabidiol oral solution, which is being studied as a possible treatment for hyperphagia and related behaviors in Prader-Willi syndrome. Click on...
Category: Research
Participate in a PWS Specific Sleep Study
Has your 3–7-year-old child been diagnosed with Prader-Willi syndrome? Do they have trouble sleeping? You may be eligible to participate in a thesis study! If your child has a confirmed diagnosis of Prader-Willi syndrome, is awake at least once per night three nights of the week and has trouble going to bed at least three...
Kasey Bedard, Ph.D., BCBA-D, IBA Shares Findings from Research Study Funded by PWSA | USA
PWSA | USA is excited to share the findings from a grant funding opportunity, awarded to and studied by Kasey Bedard, Ph.D., BCBA-D, IBA. This grant assisted Kasey with her work on PWS Smart-Start, a behavior-analytic caregiver training program. Kasey gives a brief overview of her results below. ------------------------------------------------------------------------------------------ Contributed by Kasey Bedard The purpose...
LEVO Therapeutics to Close PWS Drug Trial, Phase III Carbetocin
Yesterday afternoon, PWSA | USA learned in a conversation with LEVO Therapeutics that the company has made the decision to close their Phase III Carbetocin study. LEVO shared they are in the process of potentially being acquired. If this happens, the new company will ultimately make the decision about how and when the next study...
Hyperphagia and How it Affects Learning
Contributed by Stacy Ward, MS Director of Family Support and Lynn Garrick, RN, BSN Medical/Research Coordinator Prader-Willi syndrome (PWS) is a rare neurodevelopmental genetic disorder that affects multiple systems in the body. There are many symptoms of PWS, including hyperphagia, behavioral challenges, hypotonia, incomplete sexual development, cognitive deficits, metabolic dysregulation, and several more. Hyperphagia is...
Clinical Trial Sites Announced for Radius Health’s RAD011 SCOUT-015 Study
Radius Health has announced the locations for its RAD011 SCOUT-015 Clinical Trials. Below, you will find a downloadable map with each participating hospital and medical center around the country. The RAD011 SCOUT-015 studies are in various stages, depending on the location, from initiated to activated and ready to enroll. You can also find a comprehensive...
Saniona Pausing All Clinical Trials for PWS Drug Tesomet Due to Funding Limitations
This morning, Saniona announced they will be voluntarily pausing all Phase 2b Clinical Trials for the drug Tesomet, which is being studied as a treatment for Prader-Willi syndrome (PWS). Saniona has explained that this pause is due to funding limitations and has nothing to do with the "safety or efficacy" of the drug itself. PWSA...
What Type of Research Matters to You?
For more than 40 years, PWSA | USA has played a critical role in sponsoring and advancing research for the benefit of our PWS community. We are excited to continue this important commitment to PWS research in ways you have always relied on (organizing scientific conferences, offering grants to clinicians, etc.). We are also eager...
Help PWS experts learn more about feeding tube use in PWS
The information below was provided by the Global PWS Registry --------------------------------------------------------------------------------------- We know feeding tubes are often used in infancy for our loved ones with PWS who have difficulty feeding in the early months after birth. If your child used a feeding tube, we are asking you to spend 10 minutes today completing the new...
Global PWS Registry Shares Latest Orthopedic Data for Individuals Living with PWS
The information below was provided by the Global PWS Registry and approved by the Institutional Review Board (IRB) -------------------------------------------------------------------------------------------- Physical activity and exercise are an important part of care for individuals with PWS. However, this can be difficult due to poor muscle tone and orthopedic issues. Many individuals require bracing, casting, and/or surgery for spinal issues....
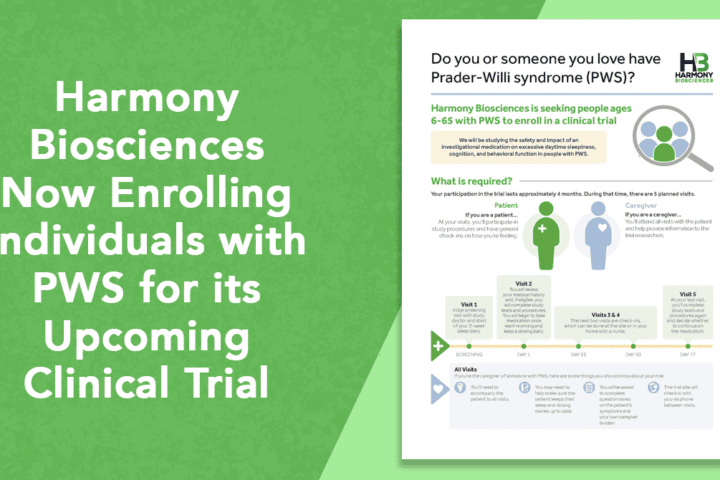
Harmony Biosciences Now Enrolling Individuals with PWS for Upcoming Clinical Trial
Harmony Biosciences has released information about their PWS Phase 2 clinical trial where they will be studying "the safety and impact of an investigational medication on excessive daytime sleepiness, cognition, and behavioral function in people with PWS." Harmony Biosciences is currently looking for individuals with PWS between the ages of 6-65 to enroll in this...
Levo Therapeutics Receives Complete Response from FDA for its Decision on Carbetocin
January 18, 2022 08:00 ET | Source: Levo Therapeutics, Inc. CHICAGO, Jan. 18, 2022 (GLOBE NEWSWIRE) -- Levo Therapeutics, Inc., a biotechnology company dedicated to using genetic insights to advance treatments for Prader-Willi syndrome (PWS) and related disorders, announced today that it has received a Complete Response Letter (CRL) from the U.S. Food and Drug Administration (FDA)...
Saniona Launches TM006 Study Website to Advance Testing Efforts for Tesomet, a Drug to Help Individuals with PWS Feel Less Hungry
Following Saniona’s announcement that the company is initiating its Phase 2b clinical trial for Tesomet, which is being studied as a treatment for hyperphagia in Prader-Willi syndrome, the company has launched a website to help advance testing efforts for the drug. If you would like to participate in PWS research for Saniona’s TM006 study, this...
National Research Study Seeks Participation from Parents of Adults with Intellectual, Developmental Disabilities
TAKE SURVEY HERE INTERESTED IN BEING INTERVIEWED? CLICK HERE DOWNLOAD FLYER HERE
Saniona Initiates Phase 2b Clinical Trial of Tesomet for Prader-Willi Syndrome
Via Saniona: PRESS RELEASE December 28, 2021 Saniona (OMX: SANION), a clinical-stage biopharmaceutical company focused on rare diseases, today announced the initiation of a Phase 2b clinical trial of Tesomet in patients with Prader-Willi syndrome (PWS). Tesomet is an investigational fixed-dose combination therapy of tesofensine, a triple monoamine reuptake inhibitor, and metoprolol, a beta-1 selective blocker. Data from the...
The Chicago School of Professional Psychology is looking for Research Study Participants
YOU can help the Chicago School of Professional Psychology learn about the effects of a behavioral caregiver training program for caregivers of children with PWS. Participants will be compensated with a $500 Visa gift card following the completion of the study. Parents with children with multiple diagnoses can reach out to Dr. Bedard for clarification...
Research Survey Opportunity: Behavioral Supports and Services for Children with Prader-Willi Syndrome
Are you a caregiver of at least one child, of any age, who has been diagnosed with Prader-Willi Syndrome? Do you have access to the internet and an internet-connected device? Can you read and write in English? Are you over the age of 18? If you answered YES to these questions, you are invited to...
Prolapsed Rectum and Risk Factors in Prader–Willi Syndrome: A Case-Based Review
Written by: Merlin G. Butler ABSTRACT A 14-year-old boy with Prader–Willi syndrome (PWS) with maternal disomy 15 is reported with rectal prolapse as only the second patient in the literature. With predisposing risk factors present for rectal damage and prolapse in this syndrome, the incidence must be higher and therefore underreported. These risk factors include...
Research Opportunity: Project Pathways
The Learning Lab for Intellectual and Developmental Disabilities at the University of Nebraska – Lincoln has reached out to us asking for any families interested in participating in a study they’re currently working on titled, Project Pathways. The purpose of this study is to assess the reading and writing profiles of students with intellectual and...
Summary of a Streamlined Molecular Diagnostic Approach for Prader-Willi and Other Related Syndromes
Written by: Merlin G. Butler, MD, PhD Historically, to confirm the diagnosis and molecular genetic classes in Prader Willi syndrome (PWS) required a stepwise approach using multiple methods needing more time and resources. Due to advances in genetic testing and availability of multiple analytical methodologies, a streamlined approach was developed and reported by Strom et...
FDA Advisory Committee to Review LEVO’s Carbetocin as a Treatment for PWS
We are excited to share that Levo’s New Drug Application (NDA) for carbetocin as a treatment for PWS will be discussed at a public meeting of the Psychopharmacologic Drugs Advisory Committee to be held November 4, 2021. This is a major step forward on this drug's path through the approval process. The Food and Drug...
Harmony Biosciences Acquires Asset with Novel Mechanism of Action for the Potential Treatment of Narcolepsy and other Rare Neurological Diseases
Harmony Biosciences recently announced the acquisition of HBS-102, a potential first-in-class molecule with a novel mechanism of action, from ConSynance Therapeutics, Inc. HBS-102 is a Melanin Concentrating Hormone Receptor 1 (MCHR1) antagonist that has the potential to offer a novel approach to the treatment of narcolepsy including the symptoms of Rapid Eye Movement (REM) sleep...
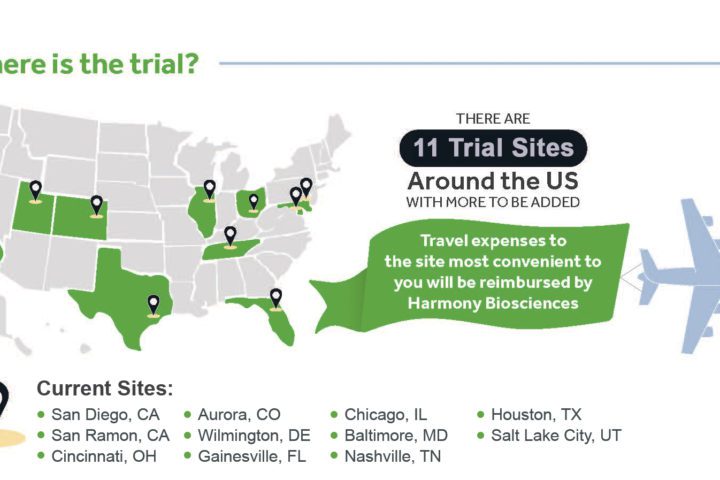
Harmony Biosciences adds Cincinnati, OH site to its Phase 2 Clinical Trial
Harmony Biosciences is currently in its Phase 2 clinical trial of Pitolisant, studying the safety and impact of an investigational medicine for excessive daytime sleepiness, cognition, and behavioral function in people with PWS. This week, they added an 11th location to their list of trial sites around the U.S. The trial site will be located...
Radius Health Announces Plans for Global Prader-Willi Syndrome Pivotal Study
On behalf of Radius Health, Inc: Boston, Mass., July 22, 2021 — Radius Health, Inc. announced Wednesday, July 21, 2021 that it has recently received the written meeting minutes from a June Type C meeting held with the U.S. Food and Drug Administration (FDA) regarding RAD011, a synthetic cannabidiol oral solution. RAD011 is initially to...





















 Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS.
Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS. Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children.
Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children. Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS.
Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS. Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts.
Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts. Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan.
Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan. Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.
Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.