Aardvark Therapeutics recently launched HERO, a global Phase 3 clinical trial investigating ARD-101, an innovative, orally administered treatment designed to help reduce hyperphagia (excessive hunger) and food-seeking behaviors in individuals with Prader-Willi syndrome (PWS). This randomized, double-blind, placebo-controlled trial is an important step toward identifying a potential new treatment option for the PWS community, and...
Category: Research
Aardvark Therapeutics Launches HERO, A Phase 3 Trial of ARD-101 for Treatment of Hyperphagia in PWS; Now Enrolling Participants in the US
Aardvark Therapeutics recently launched HERO, a global Phase 3 randomized, double-blind, placebo-controlled clinical trial of ARD-101. ARD-101 is a novel, orally administered investigational therapy being studied to see if it can reduce excessive hunger and food-seeking behaviors in individuals with PWS. About the HERO Trial As part of the HERO study, participants will be randomly...
Unlocking a New Path to Treat Hyperphagia in PWS: A Conversation with Aardvark Therapeutics
Hyperphagia, the relentless hunger that those living with Prader-Willi syndrome (PWS) experience, remains one of the most challenging and life-altering symptoms for individuals and families. But a promising investigational drug called ARD-101 is offering hope. In our April 29th episode of PWS United, Aardvark Therapeutics’ Dr. Tien Lee, M.D., CEO and Founder, and Dr. Manasi...
Request for Prader-Willi Syndrome Research Grant Applications
The Prader-Willi Syndrome Association | USA (PWSA | USA) is a nonprofit organization formed in 1975 to enhance the quality of life of those affected by Prader-Willi syndrome (PWS) through research, family support, and advocacy. The purpose of this request is to solicit applications for research projects whose findings will directly impact individuals living with...
Prader-Willi Syndrome Clinical Scholarship Announcement
The Prader-Willi Syndrome Association | USA (PWSA | USA) is a nonprofit organization formed in 1975 to enhance the quality of life of those affected by Prader-Willi syndrome (PWS) through research, family support, and advocacy. We are proud to offer scholarships of up to $25,000 USD to support providers in enhancing their understanding of Prader-Willi...
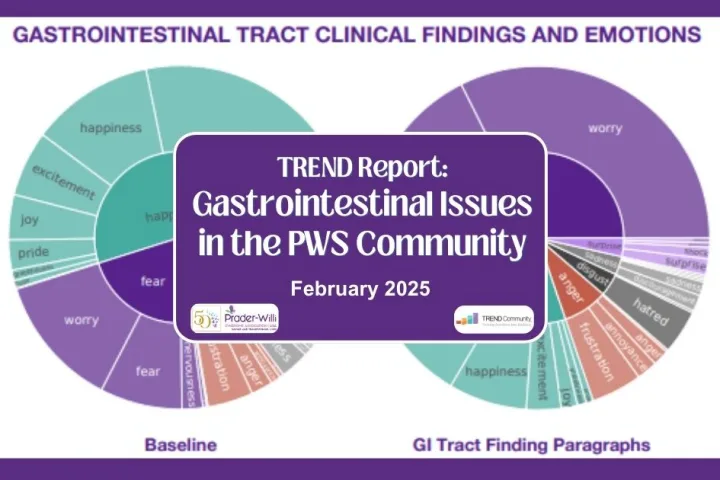
TREND Report: Gastrointestinal Issues in the PWS Community
TREND Community released its latest report on Gastrointestinal Issue in the PWS Community. This report expresses some of the most common topics involving GI issues, including constipation and bowel movements, vomiting/spitting up/gagging, prune juice and other foods, reflux, and community support. This report looked at the conversations to determine different emotions in relation to GI...
New Project Seeks to Identify Characteristics that Predict Challenging Behaviors in PWS
Individuals with PWS often exhibit rigidity, obsessive-compulsive tendencies, emotional outbursts, as well as unusual thoughts and behaviors. However, the onset and severity of these behaviors vary among individuals with PWS depending on age, genetic subtype, and other factors. A new study by Dr. David Evans at Bucknell University aims to identify early risk factors for...
TREND Pulse Report: PWS and Emotional and Behavioral Patterns
TREND Community released its latest report on PWS and Emotional and Behavioral Patterns. This report analyzed conversations parents and caregivers were having on the topic of emotional and behavioral issues. In a testament to the dedication and connection of parents to their loved ones, the largest primary emotion category was “Happiness”, with “Love” being the...
Trial Transitions and Testimonials
A month ago, Freya transitioned from the 77-day double-blind portion of the Pitolisant trial to the open-label extension, which will last at least a year or as long as she is willing to participate – whichever is longer. If you’ve been following this blog series, you know I went into this with feelings of trepidation...
The (Sometimes Messy) Details of Life in a Clinical Trial
contributed by Anne Fricke, mom to Freya (13, living with Prader-Willi Syndrome) Freya’s third and fourth appointments for the Harmony TEMPO PWS trial to study whether pitolisant is an effective treatment for excessive daytime sleepiness (ESD) in individuals with PWS, were simply routine and a lot like the second appointment. Lab work, caregiver questionnaires, counting...
Letter to Community on FDA’s Extension of DCCR Review
Dear PWS Families, We understand that hearing about the FDA’s extension of the review period for DCCR (diazoxide choline controlled release) may bring a mix of emotions, from hope to concern. Please know that this is a normal and expected part of the FDA’s thorough process, especially for a rare disease medication that has been...
FDA Extends Review Period for DCCR: What It Means for the PWS Community
On Tuesday, November 26, 2024, Soleno Therapeutics shared an important development regarding the New Drug Application (NDA) for DCCR (diazoxide choline) extended-release tablets—a potential treatment for individuals with Prader-Willi syndrome (PWS) aged four and older who experience hyperphagia. The U.S. Food and Drug Administration (FDA) has extended the review period for this NDA, pushing the...
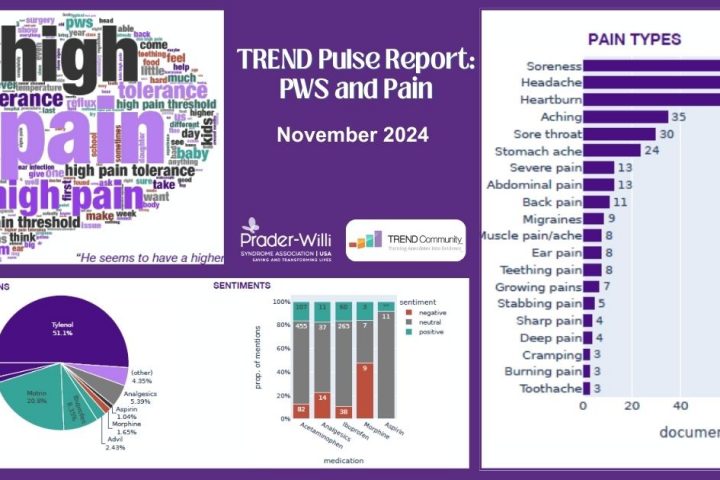
TREND Pulse Report: PWS and Pain
We are excited to share the next PWS Pulse Report that focuses on pain. TREND looked at the most common types of pain and the affected body parts. TREND also analyzed the types of pain medications discussed as well as the associated sentiments. Finally, they explored the experience of reduced pain sensitivity in individuals with...
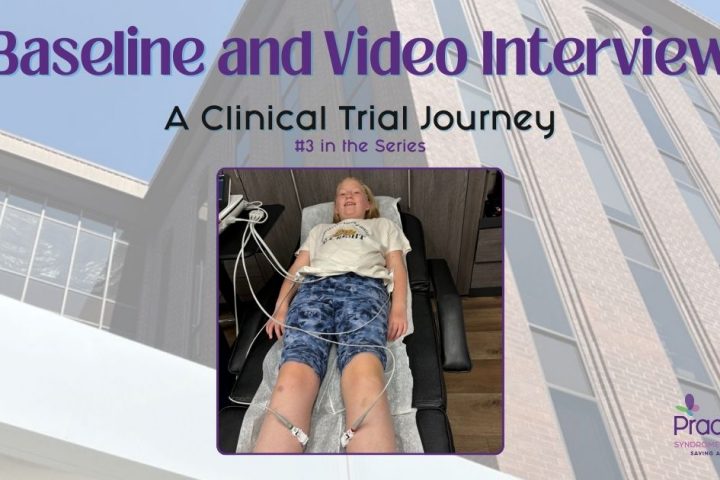
Baseline and Video Interview
contributed by Anne Fricke (This post picks up from “The Screening Appointment” if you would like to start there.) Baseline Appointment (1.5 hours, on-site): Spending a night in southern California is not my idea of fun or relaxation. During our first trip, Freya enjoyed the arcade on the pier and splashing in a slightly warmer...
A Parent’s Perspective on their Child’s Clinical Trial
contributed by Susan Fries, mom to Roselyn, 7-year-old living with PWS “I feel like we are all waiting for that magic fix, and if it works for someone else then my kid must drink the kool-aid and it’ll work for them too. But we deep down know it doesn’t work that way as much as...
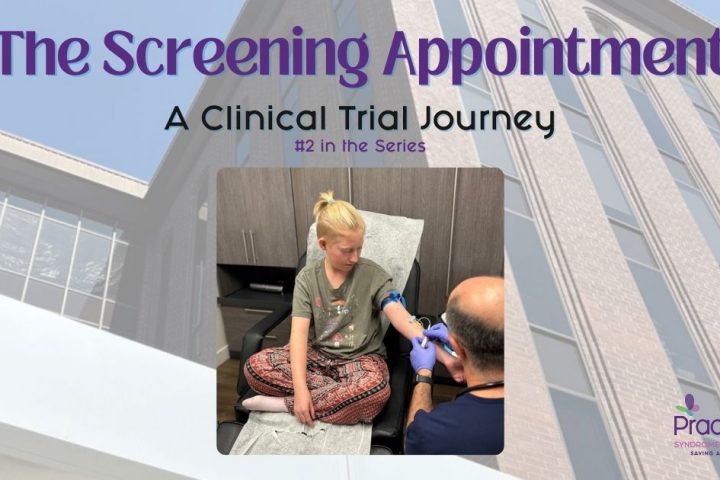
The Screening Appointment
contributed by Anne Fricke The intention of this series is to shed light on the process of enrolling and participating in a clinical trial, as well as to create a space to openly share the many emotions that are involved when a family decides whether or not to join. Part of the decision-making process should...
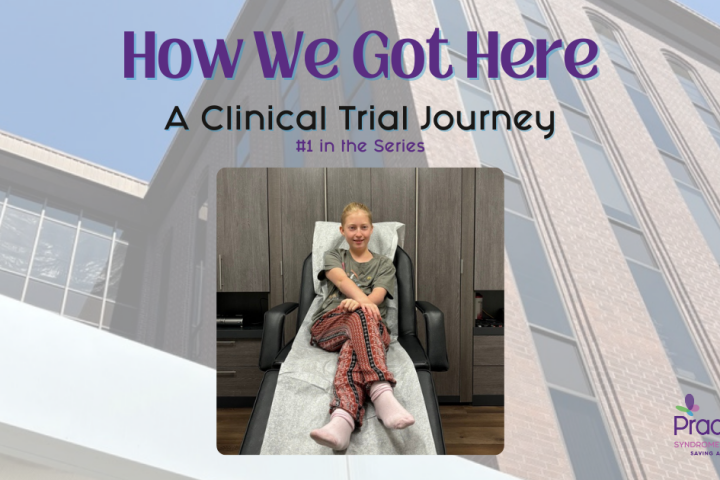
How We Got Here
contributed by Anne Fricke I lay awake in a pre-dawn haze the morning Freya was to take her first pill. We had been to Southern California twice already, a combination of 7 flights up and down the coastline, numerous hours of travel, and far too many airport meals, and I was still momentarily on the...
Update on Phase 3 COMPASS PWS Study from Acadia
September 24, 2024 Dear Prader-Willi Syndrome Community, We are pleased to share an update on the 12-week, pivotal Phase 3 COMPASS PWS study evaluating the efficacy and safety of carbetocin nasal spray (ACP-101), an investigational drug, for the treatment of hyperphagia in Prader-Willi syndrome. The study was initiated in the United States in November 2023...
FDA Advisory Committee to Review DCCR as Treatment for Hyperphagia in PWS
The FDA has announced its plan to conduct an Advisory Committee Meeting as part of its review of Soleno Therapeutics’ DCCR New Drug Application. The FDA convenes Advisory Committees to provide independent expert advice that contributes to the agency’s regulatory decision-making. As part of the Advisory Committee Meeting, interested community members are encouraged to share...
FDA Accepts Application for New Drug DCCR, Moves to Priority Review
Exciting news for the Prader-Willi syndrome (PWS) community! Soleno Therapeutics has announced that the FDA has accepted their new drug application (NDA) for DCCR, a drug designed to treat hyperphagia in individuals with PWS aged 4 and older. This acceptance is a major first step, and the FDA has granted Priority Review, recognizing the potential...
Survey Results on the Aging Adult with PWS
Contributed by Barb Dorn, RN, BSN As I began my research looking at specific health issues in the aging adult with PWS, I soon learned that there was not much information on this topic. I did find a few articles that documented clinical evidence for early signs of aging. As far as dementia, I found...
Aging Research in Prader-Willi Syndrome
Compiled by Barb Dorn, RN, BSN People with PWS are growing old. Many of this may be the result of our increased knowledge in supporting and caring for the person with PWS. We have learned to replenish hormone deficiencies and manage their diet and food security. We have identified critical health issues and know that...
Soleno Therapeutics Submits New Drug Application to FDA for PWS Treatment
On June 28, 2024, Soleno Therapeutics announced the company officially submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for DCCR (diazoxide choline) extended-release tablets. This new treatment targets Prader-Willi syndrome (PWS) in individuals aged 4 and older with hyperphagia. CEO Anish Bhatnagar, M.D., says, “Submission of the DCCR NDA to...
Help Needed: Caregivers of Children with Prader-Willi Syndrome and Repetitive Verbal Behavior
Kasey Bedard, PhD, and her team at The Chicago School are seeking caregivers of children diagnosed with Prader-Willi Syndrome (PWS) who exhibit repetitive verbal behavior for a research study at The Chicago School. This study, part of a dissertation project, aims to test interventions that caregivers can implement at home. Study Details: – Duration: 2-3...




















 Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS.
Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS. Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children.
Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children. Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS.
Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS. Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts.
Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts. Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan.
Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan. Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.
Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.