PWSA | USA (Prader-Willi Syndrome Association | USA) and FPWR (Foundation for Prader-Willi Research) are thrilled to announce a groundbreaking collaboration to address the social challenges faced by individuals with Prader-Willi syndrome (PWS). FPWR funding has facilitated the development of the Building Our Social Skills (BOSS) curriculum, a highly effective social skills intervention program created...
Tips and Techniques for a Safe Holiday Season
Whatever holiday you and your family celebrate, PWSA | USA wants to help you make it both fun and safe. The key is to plan well ahead of time, incorporating the strategies outlined in this article. Celebrate Winter Holidays Safely Many of these tips can carry over to other holidays celebrated during the winter months Replace those...
Healthy Holiday Recipes
Silly Apple Monsters Ingredients: 3 green apples 3 tablespoons creamy peanut butter 2 ounces of sliced cheese (cheddar or colby jack both work) 2 tablespoons of pumpkin or sunflower seeds A package of candy eyes Instructions: Quarter the green apples and slice off the core and seeds. Carefully cut out a v-shape into the center...
Celebrate Thanksgiving Safely
by Katherine Crawford, former PWCF Family Support Coordinator Updated by Lisa Graziano, M.A., PWCF Education and Training Consultant Holidays are a time for gathering, connecting, and celebrating – but are also typically centered on food, which often places enormous stressors on families of a child or adult with PWS. We hope the following tips make...
Soleno Therapeutics Reports Positive Results from DCCR Study C602 for Prader-Willi Syndrome
Soleno Therapeutics, Inc. has revealed positive outcomes from the randomized withdrawal phase of Study C602, an extended treatment study of DCCR (Diazoxide Choline) Extended-Release tablets for Prader-Willi syndrome (PWS). The results, which showed a significant improvement in hyperphagia-related behaviors in the DCCR group compared to the placebo group, support Soleno's plan to submit a New...
Calling All New Englanders! Support PWSA | USA at the 14th Annual Hunter Lens Golf Tournament
Calling all New Englanders - Join the Lens family for a full afternoon of fun activities! The 14th Annual Hunter Lens Golf Tournament will take place Saturday, October 7, 2023, at 12:00 p.m. EST at the Back Nine Club (17 Heritage Hill Dr., Lakeville, MA 02347). Enjoy time with family and friends while participating in...
Transformative Tales: Empowering Families Dealing with Prader-Willi Syndrome Through Food Security
Contributed by Christopher Rich, Utah PWS Association Living with Prader-Willi Syndrome (PWS) presents unique challenges, particularly in managing food-related behaviors. The experiences of families dealing with PWS takes on an extra layer of complexity, because of the significance of food control and security devices in their lives. In the below excerpts, we dive into those...
Neuren Pharmaceuticals is Happy to Announce the First Site Participating in Their Phase II, Open Label, PWS Study (Neu-2591-PWS-001) is Now Open for Screening!
Important information regarding this exciting milestone: Rare Disease Research (RDR), located in Atlanta, GA, is now welcoming children with PWS and their families to their clinicfor screening into this trial. The duration of active treatment in this study is 13 weeks. In a preclinical study in animals, physiological and behavioral symptoms were normalized within six...
Harmony Biosciences Issues Statement Regarding Confidence in Pitolisant Drug
Harmony Biosciences has reaffirmed its confidence in the strength of WAKIX® (Pitolisant) patents, after receiving a positive ruling from the U.S. Patent and Trademark Office (USPTO) rejecting the request for reexamination. WAKIX® is used to treat excessive daytime sleepiness (EDS) or cataplexy in adults with Narcolepsy. Read Harmony Biosciences' community-facing statement below: We are pleased...
2023 Moms’ Retreat Attendees Selected!
Thank you to everyone who submitted an application to attend PWSA | USA's first-ever Moms' Retreat, October 12-15, 2023, in Palm Spring, California! On Friday, August 18, 2023, 30 applicants were randomly selected to attend the event. Our staff will be reaching out to these individuals to provide additional details and collect information. Below you...
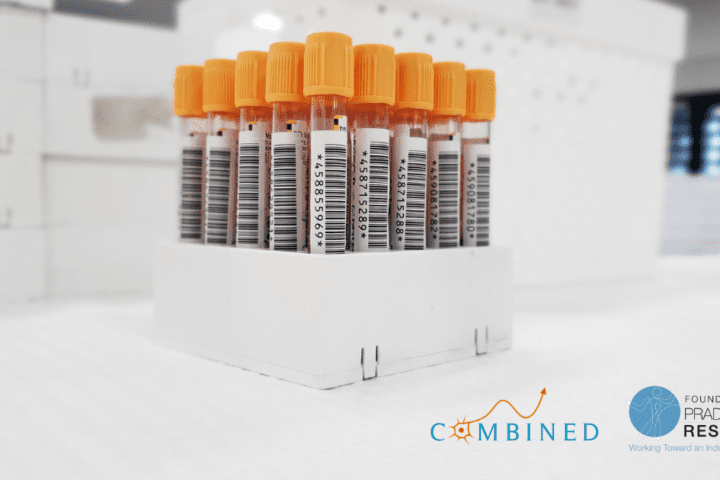
Help Rare Disease Research Efforts by Sharing Blood, Urine Samples with PWS-COMBINEDBrain Biorepository
We are sharing details on behalf of The Foundation for Prader-Willi Research (FPWR) to spread the word about an upcoming and important PWS research opportunity. FPWR is collaborating with COMBINEDBrain to establish a biorepository dedicated to blood and urine samples. This initiative aims to expedite the development of treatments for individuals with Prader-Willi Syndrome, as...
Aardvark Therapeutics Announces FDA Pediatric Disease Designation for PWS, Expansion of Phase 2 Clinical Trial
Aardvark Therapeutics recently announced the drug company has received Pediatric Disease Designation for PWS from the U.S. Food and Drug Administration (FDA), and will expand its Phase 2 clinical trial of oral ARD-101 in young adults with PWS. According to Aardvark Therapeutics, this FDA designation means the company is eligible for a Rare Pediatric Disease...
Get to Know PWSA Egypt and Middle East!
Individuals and families affected by PWS who are living in Egypt and the Middle East now have an established community to find help and hope. PWSA Egypt and Middle East was officially founded on June 1, 2023 by Walaa Mohamed, mom to Ahmed, 13, living with PWS. PWSA Egypt & Middle East will serve the...
What is Disenfranchised Grief and How Can PWSA | USA Offer Support?
Contributed by PWSA | USA Alterman Family Support Counselor Kim Tula, MS, CSW Grief is the response to loss, particularly to the loss of someone or some living thing that has died, to which a bond or affection was formed. But what about the feelings of loss associated with living with PWS? Is this a...
PWS Hope United Spotlight: Kissing for a Cause
Mr. & Mrs. Steven & Tara Davis of Wilbraham, Massachusetts tied the knot this past weekend and celebrated their love by "Kissing for a Cause" during the reception! As a long-time friend of Melanie McDonald, mom to Josephine, 5, living with PWS, Tara wanted to raise money for PWSA | USA in honor of Josephine...
How Utah is Enhancing Food Security for Prader-Willi Syndrome Individuals: 2023 PWS National Convention
Contributed by Christopher Rich, Utah PWS Association Among the exhibitors at this year's PWSA | USA National Convention was the Utah PWS Association, which focused on food security and its role in managing PWS symptoms. The exhibition booth showcased practical strategies and innovative solutions to create a controlled environment, emphasizing the importance of food security...
Share Your School Lunch Tips
On Friday, July 28, 2023, PWSA | USA's Special Edition Pulse will focus on back-to-school and offer helpful tips from our Family Support team as well as how to best utilize our School Success resources. We also want to hear from YOU, our PWS community, on what has worked for you / your loved one...
Harmony Biosciences Announces Plan to Begin Phase 3 Trial for Pitolisant Following Meeting with FDA
PWSA | USA received news from Harmony Biosciences that the company is working on a plan to begin their Phase 3 clinical trial for pitolisant, also known as WAKIX, following a positive End-of-Phase 2 meeting with the U.S. Food and Drug Administration. WAKIX is an FDA approved drug used to treat excessive daytime sleepiness or...
Gedeon Richter Now Recruiting for KITE-PWS Clinical Trial
You or someone you love could be part of developing new therapies for Prader-Willi syndrome. Learn about Gedeon Richter's research study KITE-PWS, also known as RGH-706-003, to evaluate an experimental drug for hyperphagia in people with Prader-Willi syndrome. The oral drug RGH-706 works by blocking melanin-concentrating hormone (MCH), which is a key part in the...
Prader-Willi Syndrome Association | USA Announces the Resignation of CEO Paige Rivard and Appointment of Interim CEO Stacy Ward
July 12, 2023 – The Prader-Willi Syndrome Association | USA (PWSA | USA) announces the resignation of its CEO, Paige Rivard, MBA. Paige held the CEO position for the past three years and has led many efforts to advance awareness, research opportunities, and provide support for families in the Prader-Willi syndrome (PWS) community. The association...
Spotlight on Advocacy: PWS Advocate Erin Cooper Carter, PhD Continues to Fight for our Ohio Families!
Erin Cooper Carter, PhD (mom to Victoria, age 6 living with PWS) is leading the effort to have PWS added to the state’s list of developmental disabilities in Ohio! This important piece of legislation is being filed by Representative Bride Rose Sweeney (D-16) and will likely be co-sponsored by Representative Nick Santucci (R-64). When asked...
Congratulations to PWSA | USA’s 2023 Volunteer Award Recipients
“Never doubt that a small group of thoughtful, committed citizens can change the world; indeed, it’s the only thing that ever has.” – Margaret Mead On June 24, 2023, the last day of PWSA | USA’s 37th National Convention, seven individuals who have gone above and beyond to not only spread the mission of PWSA...
PWSA | USA’s Hope United Gala Silent Auction is Now LIVE!
Anyone can bid on our amazing Hope United Gala Auction items! Click the images below to check out just a few of the 40+ experiences, sports memorabilia, jewelry, baskets, and more. There's truly something for everyone to enjoy.
Acadia Pharmaceuticals Announces Next Phase for ACP-101 (Intranasal Carbetocin) After Meeting with FDA
This week, Acadia Pharmaceuticals made the announcement the drug company plans to begin Phase 3 of ACP-101, intranasal carbetocin, later this year after a recent meeting with the U.S. Food and Drug Administration (FDA). ACP-101 is currently being studied to treat hyperphagia in Prader-Willi syndrome. Learn more about this announcement by reading Acadia Pharmaceuticals’ full...





















 Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS.
Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS. Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children.
Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children. Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS.
Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS. Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts.
Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts. Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan.
Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan. Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.
Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.