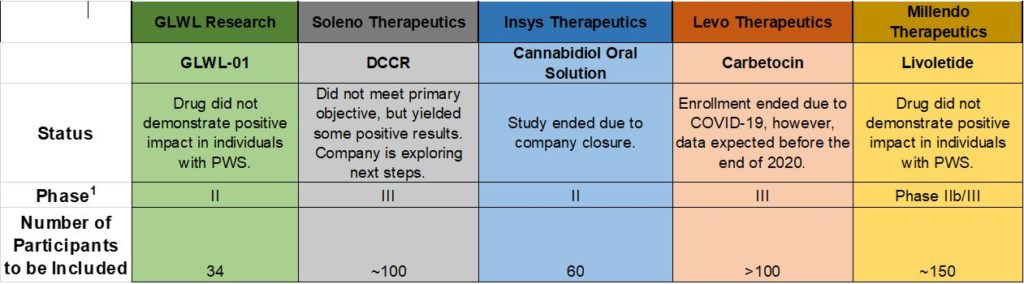
Approximately two years ago, several companies began clinical trials of drugs with the potential to treat hyperphagia and associated behaviors in Prader Willi syndrome (PWS). Outcomes of four of five trials are now available.
Soleno Therapeutics recently announced the results of clinical trials of DCCR. In the double-blind study, caregivers were asked to rate the impact of either DCCR or a placebo on their loved one’s hyperphagia (caregivers did not know if their loved one received the drug or a placebo). Though DCCR’s impact was not statistically significant across all subjects in the study, a significant drop in excessive appetite was observed in a subgroup of patients with more severe hyperphagia. DCCR treatment was also noted to result in significant clinical improvements, as assessed by the investigators, and a significant drop in body fat, compared with the placebo.
Levo Therapeutics continues to run their trial of carbetocin (which is similar to oxytocin). Levo recently announced that it has decided to close enrollment due to the COVID-19 pandemic and will be announcing results of the study later this year.
Two other drug programs have ended as the drugs studied did not prove to have a significant impact in those with PWS. Another ended as the developer has gone out of business.
While the news on these clinical trials is mixed, there were positives and hope remains.
- DCCR did show positive signs it worked in PWS and might be able to get to the market in the future.
- Levo Therapeutics could announce positive results for carbetocin later this year.
- There are other programs in development that could advance forward in the future.
- As a community, we helped to complete these trials and the companies involved learned how to conduct them. This will be important for future clinical trials as other companies considering pursuing trials for PWS. We should be proud.
- Multiple companies saw the value of pursuing a therapy for PWS paving the way for more in the future.
- We learned that we need to further refine how we measure hyperphagia in the future.
Below is an updated table showing the status of the five trials:
Footnotes:
Drugs in development go through three stages called Phase 1, Phase 2 and Phase 3. Phase 1 is the first phase of in human tests and is usually done in healthy volunteers. Phase II usually include treatment in patients to judge efficacy and is often done to determine dosing. Phase 3 is the stage needing the most patients and lasts the longest. It is the typically done with randomized, placebo-controlled trials to finalize the testing before FDA review.
Contributed by Rob Lutz




 Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children.
Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children. Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS.
Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS. Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts.
Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts. Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS.
Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS. Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan.
Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan. Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.
Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.