The recent webinar: How Patients Can Partner to Speed Treatments for PWS, co-presented by FPWR and PWSA-USA, is now available. This webinar provides important information about PWS clinical trials including: what to expect when participating in a clinical trial, what questions you should ask, and how to get the information you need to decide if a clinical trial is right for your loved one with PWS. The webinar also reviews current and upcoming PWS trials.
What is a clinical trial and why is it important?
In order to determine if a drug treatment is both safe and effective, the FDA requires that the drug owner conduct a wide range of tests over many years. Tests in humans are often called “clinical trials.” Clinical trials typically involve having participants (or subjects) agree to take a new drug (or a placebo) and to have their health monitored to see the effects of a drug. See the Clinical Trial Glossary and Background below to learn more.
Clinical trials are critically important for the PWS community because they allow scientists to understand more about potential treatments for PWS. Only through the data collected from clinical trials can new PWS treatments be developed and offered to everyone in the community.
That said, clinical trials have risks and requirements that one should consider carefully before enrolling. And always consult with a physician before enrolling in a clinical trial.
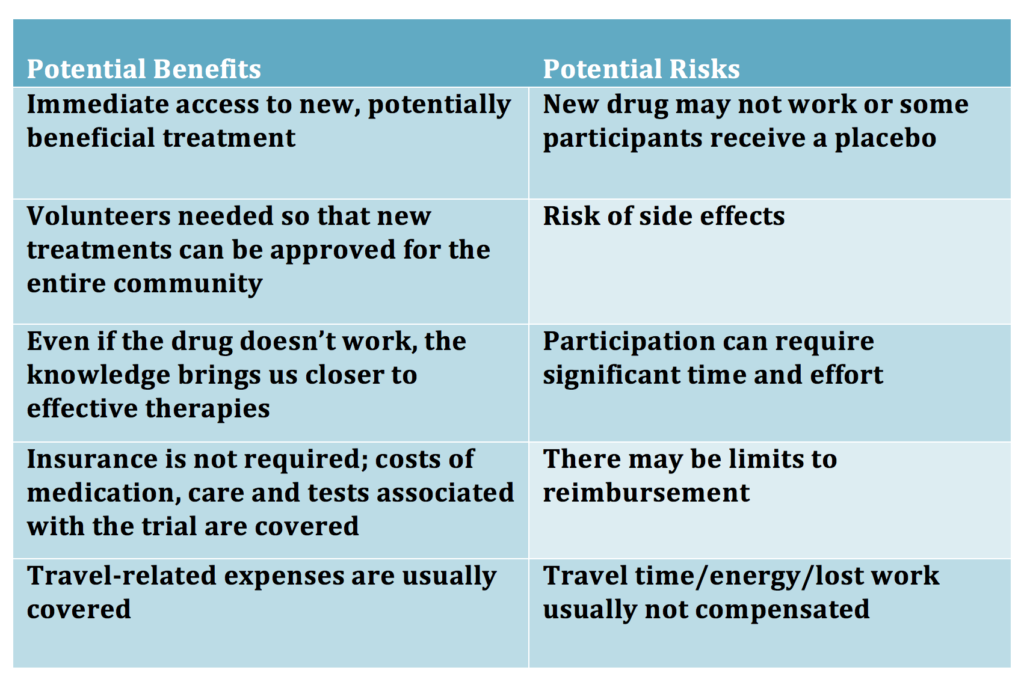
Should I enroll my loved one with PWS in a clinical trial?
There are many factors to consider before enrolling in a clinical trial. The table below is a high-level summary of some factors to consider.
What are the steps in participating in a clinical trial?
- Screening—this step is an evaluation to determine if a volunteer can be included in the trial based on their demographics, particular characteristic (genetic subtype, clinical features, etc.), health, and other drugs being taken (that might interact with the study drug)
- Consent—during which a participant and/or guardian agrees to participate after gaining sufficient knowledge about the study and having all their questions answered
- Study Schedule– a required series of visit to the physician to perform an examination and to track a variety of health metrics used to evaluate the safety and efficacy of the drug. Usually the drug (or placebo) is provided after the initial baseline visit. The visits tend to be frequent in the beginning and then slow down
- Follow up / reporting—months after participation, results of the study usually become available either in medical journal or on ClinicalTrials.gov or in a report to the community via webinar or meetings
Will a participant always receive the active drug or could he/she get a placebo (fake drug)?
All clinical trials are different. However, in many clinical trials, participants could receive placebo either for all or part of a trial. This is done to provide a “control” to make sure that the effect of the active drug is real compared to a fake.
In some trials with “parallel arms” (see glossary below), some patient will randomly be selected to receive placebo. In other trials (crossover trials) all patients receive both active drug and placebo bit they don’t know in which order.
Many studies have an “open label extension” where all trial participants (after an initial period when they might receive placebo) receive the active drug. If a trial has an open label extension, all participants will have the option to receive active drug during this phase.
Where can I get up to date information about PWS clinical trials?
Both PWSA(USA) and FPWR have information about current clinical trials on their websites, see https://www.pwsausa.org/research-announcements/and https://research.fpwr.org/pws-clinical-trials
You can also find the official record of the clinical trial, provided by the sponsoring pharmaceutical company or university, on www.clinicaltrials.gov; search for Prader-Willi syndrome:
You can also sign up for a monthly “Clinical Trial Alert” at and https://research.fpwr.org/pws-clinical-trials to get up to date information about new trial openings and new trial sites.
Finally, if you join the Global PWS Registry (www.pwsregistry.org) and click the button to be contacted for clinical trials, we will let you know when there is a trial that you might be eligible for, and provide you with contact information for the study.
What questions should I ask before enrolling a clinical trial?
Some basic information about clinical trials, including ‘what to ask’, can be found on the following websites:
https://www.nih.gov/health-information/nih-clinical-research-trials-you/basics
https://clinicaltrials.gov/ct2/about-studies/learn
https://www.ciscrp.org/education-center/ (more patient friendly)
Who should I talk to about participating in a clinical trial to get more information?
- Your doctor– the doctor or medical professional who follows your loved one with PWS closely is a good first person with whom to have a discussion about participation in clinical trials. They may not have all of the details on any specific clinical trial, but can help you sort through possibilities.
- Study Investigator at the site– the doctor in charge of the trial at the clinical trial site will have all of the background information on the drug and the trial. They should understand all of the known risks and benefits, and can advise you on whether the drug is potentially a good ‘fit’ for your loved one with PWS.
- Study Coordinator at the site– this is usually the contact person on the clinical trial record. They are not the doctor but will know all the logistics of the trial (eligibility, number of visits, procedures, etc.).
- IRB Director: each clinical trial site is overseen by an “Institutional Review Board”. If there are any concerns about the way the trial is being conducted, the IRB Director can help.
Where can I find out about other clinical research opportunities that may not involve drugs (eg, studies evaluating behavioral interventions, web-based studies, etc)?
The site: https://research.fpwr.org/pws-clinical-trials includes non-drug studies that might be of interest. These studies are also included in the Clinical Trial Alert.
Clinical Trial Glossary and Background
Drugs in development go through three stages prior to approval, called Phase 1, Phase 2 and Phase 3.
- Phase 1 is the first phase of in human tests and is usually done in healthy volunteers and is focused mainly on determining safety.
- Phase 2 usually includes treatment in patients to judge efficacy and is often done to determine dosing. Safety is also monitored.
- Phase 3 is the stage needing the most patients and lasts the longest. It is the typically done with randomized, placebo-controlled trials to establish safety and efficacy before FDA review.
Placebo—a “fake” drug that looks like the active drug but has no medicine. Placebo is given to compare results for an active drug to a fake drug to see if the active drug is actually making a difference.
Randomized— this refers to a trial that randomly chooses which patients are on active drug and which are on placebo.
Crossover study—is a study in which trial participants go thoruhg two phases of testing—one on active drug and one on placebo. It is randomized whether one is put on active drug or on placebo first
Parallel arm study— it is randomized whether one is put on placebo or on active drug
Blinded (single, double or triple)—to avoid bias, trials often prevent patients, doctors and evaluators from knowing who has received active drug or placebo. This is referred to as blinding or a blinded trial.
- In a single blind study the patient does not know if he/she is receiving active drug or placebo.
- In a double blind study, neither doctor nor patient knows who is getting drug or placebo.
- With a triple blind trial study, neither the patient, the doctor or the person who evaluates response know who is getting active drug and who is getting placebo.
Open label study— an open label study is not blinded and there is no one taking placebo. Everyone knows that the patient is receiving the active drug.
Open label extension – in some clinical trials, there is a time after the randomized, blinded period in which all participants are allowed to receive the active drug if they choose. This can be good for patients as it allows them to receive or continue an active drug, and it is good for the owner as they can gain more information about longer term safety and efficacy. This period is known as “open label” since everyone knows the patient is on drug (ie, it is no longer blinded)
Several valuable resources were shared during the webinar and are listed below:
PWSA-USA: Active Clinical Trials of PWS Hyperphagia
: compares 5 PWS clinical trials for hyperphagia using information collected from the sponsoring companies. Information provided includes company name, phase, type of study, administration, eligibility criteria and more.
FPWR: PWS Clinical Trials in Progress and Starting Soon: provides a high level view of current PWS drug and intervention studies with age eligibility, trial sites, BMI requirements, etc., as well as links to more information on each trial. Information is updated regularly to ensure data is up-to-date and reliable.
Global PWS Registry: the Registry provides critical support for PWS clinical trials. If you have a loved one with PWS, we invite you to enroll in the registry and update your surveys annually.
ClinicalTrials.gov: a database of privately and publicly funded clinical studies conducted around the world. Includes detailed trial information including eligibility criteria, trial design and trial sites.





 Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS.
Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS. Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children.
Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children. Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS.
Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS. Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts.
Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts. Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan.
Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan. Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.
Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.