
Prader-Willi Syndrome Association | USA
Empowering Individuals, Supporting Families
PWSA | USA's 24-Hour Crisis Phone Line: (941) 312-0400
PWSA | USA’s 24-Hour Crisis Line provides immediate, expert support to families facing medical or behavioral emergencies, ensuring you are never alone during critical moments. Available 24 hours a day, every day of the year (including holidays), our knowledgeable Family Support Team is always on the other end of the line, ready to listen and help. You can also email our team at info@pwsausa.org.
PWS Advocacy & Awareness
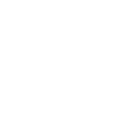
PWS Family Support
PWSA | USA’ Family Support team provides individuals diagnosed with Prader-Willi syndrome, their families, and care providers with critical information and resources on PWS.

PWS Research
PWSA | USA seeks to support research projects with the potential for immediate and high impact for the Prader-Willi Syndrome community.

We look forward to seeing our community at the 2025 United in Hope PWS Conference!
Registration is now CLOSED for the United in Hope Conference. For attendees: Click the button below to find helpful information ahead of the event.
June 24-28, 2025 | Phoenix, Arizona


Celebrating 50 Years of Support, Advocacy, and Progress
New Diagnosis?
Fill a New Diagnosis form to receive our Package of Hope, a Parent’s Guide to Prader-Willi Syndrome.
Click Here

Join our Newsletter
Join our mailing list for Prader-Willi Syndrome Association updates and other relevant information.
Sign Up

PWS United Podcast
A podcast produced by PWSA | USA for the Prader-Willi syndrome community! Find PWS United on Apple Podcasts, Spotify, or anywhere you listen to podcasts.
Listen Now
May is PWS Awareness Month!
The month of May is an important time for our rare disease community because it's recognized as Prader-Willi Syndrome Awareness Month. While advocacy efforts, the fight for research advancements, and the celebration of our loved ones are important 365 days a year, the 31 days in May offer an opportunity to really show off our PWS pride. Find several different ways YOU can make an impact by clicking the button below!
PWS Awareness Month Hub
Join the PWS Connect Community & Research Initiative

Become a part of the PWS Connect Community to help accelerate scientific progress for people living with Prader-Willi syndrome (PWS).
Join the Initiative Today!
Discover the Vital Role of Growth Hormone in PWS Care
Explore PWSA | USA’s comprehensive Growth Hormone Booklet, a critical resource for families, doctors, and healthcare professionals.
Growth Hormone Booklet
PWS Events & Fundraisers
Upcoming Events
To Register as a GOLFER or a TEAM click here Join us for a day on the links on Saturday, September 6, 2025 at Heron Glen Golf Course in Ringoes, NJ in remembrance of Jim Worthington. A portion of the proceeds will be donated to PWSA, in Jim's name. This event is inclusive for golfers […]

Lakeville, MA 02347 United States
LEARN MORE AND REGISTER TO ATTEND HERE When: Saturday, September 13, 2025 | 1:00 PM ET Where: Heritage Hills Golf Club, 17 Heritage Hill Drive, Lakeville, MA Join the Lens family for a day of golf, good spirits, and great friends as you golf and dine at Heritage Hills Golf Club in Lakeville, MA. This […]

St. Louis, MO 63105 United States
50th Anniversary Celebration: Journey of Hope Gala CLICK HERE TO PURCHASE TICKETS Join PWSA | USA as we celebrate 50 years of support, research, and community at our Journey of Hope Gala. Date: Friday, September 26, 2025, from 6 PM - 10 PM Location: Ritz Carlton, St. Louis, MO Together, we will reflect on the […]

Visit Our Blog
Aardvark Therapeutics HERO Trial: U.S. Sites Now Open
Aardvark Therapeutics recently launched HERO, a global Phase 3 clinical trial investigating ARD-101, an innovative, orally…
Spotlight on Hope: Ada Thrives and Shines
submitted by Caitlin Heckman, mom to Ada (1, living with PWS) We received Ada’s PWS diagnosis…
Aardvark Therapeutics Launches HERO, A Phase 3 Trial of ARD-101 for Treatment of Hyperphagia in PWS; Now Enrolling Participants in the US
Aardvark Therapeutics recently launched HERO, a global Phase 3 randomized, double-blind, placebo-controlled clinical trial of ARD-101.…
Spotlight on Hope: Lydia and Dalyas Dreamers
PWS Mom Jamie Caldwell, has found a unique way to celebrate her daughter, Lydia (3, living…
Spotlight on Hope: Mastering Karate with Cameron
submitted by Lisa Graziano, proud mom to Cameron (26, living with PWS) Cameron Graziano, age 26…
VYKAT XR Town Hall Summary
On Tuesday, April 22, PWSA | USA and FPWR offered a PWS Community Town Hall for…
Giving HOPECreating IMPACT
Be a part of something great. We are utterly dedicated to giving hope to those in need, creating a lasting impact for them.









 Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS.
Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS. Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children.
Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children. Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS.
Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS. Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts.
Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts. Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan.
Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan. Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.
Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.